What is immunogenicity?
Immunogenicity refers to the ability of a substance to trigger an adaptive immune response in the body. It is characterized by the presence of anti-drug antibodies (ADAs) — antibodies that bind to the idiotope of another antibody, protein, etc, in a patient or animal following biopharmaceutical administration.
How does immunogenicity arise?
Immunogenicity can arise from two main mechanisms. The first is T-cell dependent, while the second is T-cell independent. The T-cell dependent pathway involves antigen-presenting cells that capture and internalize antigens. These antigens are then processed and displayed on the surface of antigen presenting cells in association with MHC class II molecules. This presentation allows T cells to recognize the antigens through their T-cell receptors, initiating an immune response. Such responses are typically robust, long-lasting and characterized by high antibody titers, especially against foreign or exogenous proteins.
In contrast, the T-cell independent pathway does not rely on T-cell involvement. Instead, antigens with specific repeating epitopes directly crosslink B cell receptors, stimulating B cells to differentiate into plasma cells and produce ADAs. This pathway is predominantly activated by particulate antigens or antigens derived from microbial or viral sources [1].
Key definition: “The propensity of the therapeutic protein product to generate immune responses to itself and to related proteins or to induce immunologically related adverse clinical events” [101].
ADAs can be categorized into neutralizing antibodies (NAbs), which bind to the biopharmaceutical’s active site and inhibit its activity, and non-neutralizing antibodies, which do not bind to the active site but may still reduce therapeutic efficacy by compromising bioavailability. All ADAs can adversely affect the pharmacokinetics (PK), pharmacodynamics (PD), bioavailability and clinical efficacy of biopharmaceuticals.
Why biopharmaceuticals?
Biopharmaceuticals are therapeutics produced through biotechnological processes using living organisms, typically through genetically engineering living bacterial, animal or plant cells [2]. They are usually large and structurally more complex than small molecule drugs, requiring highly specialized techniques and lengthy production times [2,3]. Among the most common biopharmaceuticals are monoclonal antibodies (mAbs), recombinant analogs of growth factors and cytokines, but can also include peptides, enzymes, growth factors, hormones and engineered proteins, among others [4]. Between January 2018 and June 2022, there were 180 distinct biopharmaceutical product approvals in the United States and Europe, demonstrating their continued growth in recent years [5].
Biopharmaceuticals are more likely to induce an immune response due to their large size and exogenous origin, i.e., they may contain neo- or non-self-antigens or may be similar to ‘self’ molecules like self-antigens. In either case, these molecules cause an activation of antibody-secreting B-cells, which leads to the clinical manifestation of immunogenicity [6,7]. Some small molecules containing proteins can also induce immunogenicity, but this is less common [102].
Desired vs undesired immunogenicity
Immunogenicity can be classified as desired or undesired [102].
Desired immunogenicity typically concerns vaccines, where pathogen-derived molecules are intended to be recognized as foreign and generate an immune response, as this builds immunity and creates protection for the patient.
As with the administration of biopharmaceuticals such as monoclonal antibodies, immunogenicity is an undesired consequence, as the foreign molecule is intended to be tolerated and not recognized as a pathogen [8,102].
Why is it important to test for immunogenicity?
Most biopharmaceuticals induce immunogenicity and while many do not induce clinically relevant immune responses, some cause severe and potentially lethal consequences, including anaphylactic reactions, circulatory failure and thromboembolic events. Unwanted immune responses can also cause a loss of efficacy of the drug, leading to autoimmunity to endogenous molecules [6].
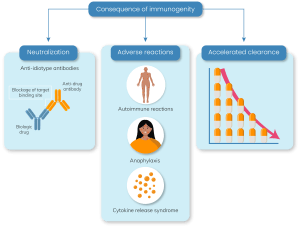
Safety:
Immunogenicity can result in serious acute immune effects such as:
- Hypersensitivity reactions (e.g., anaphylaxis)
- Immune complex disease
- Cross reaction with native proteins or receptors
- Allergic reactions
- Severe autoimmune reactions
- Cytokine release syndrome [102]
Efficacy:
Production of ADAs can also play a role in altering the efficacy of a drug by either accelerating or impeding the drug’s clearance [9]. ADAs that bind the same epitope as the target have neutralizing properties and block the pharmacological function of the drug. In addition, ADAs can lead to the formation of immune complexes, which are associated with accelerated drug clearance or extended half-life, and may also influence the clinical response to biopharmaceutical treatment through sub-optimal drug levels [10]. Therefore, these safety and efficacy side effects render the assessment of immunogenicity a crucial part of drug safety evaluation.
Factors impacting immunogenicity
Many patient-, disease- and product-related factors can influence the immunogenicity of therapeutic proteins.
![]() Patient-related factors: Genetic background, prior exposure, pre-existing immunity, immune status (including immunomodulating therapy), and concomitant illnesses, notably of the kidney and liver, [3,6,102].
Patient-related factors: Genetic background, prior exposure, pre-existing immunity, immune status (including immunomodulating therapy), and concomitant illnesses, notably of the kidney and liver, [3,6,102].
![]()
Treatment-related factors: Dosing schedule, frequency and route of administration, drug mechanism of action and therapy duration.
![]() Product-related factors: Structural properties of the biopharmaceutical (e.g., protein sequence), presence of exogenous and endogenous epitopes, degree of glycosylation, exposure of antigenic sites, formulation and storage, down-stream processing, impurities/contaminants, solubility and stability, among others [3,6].
Product-related factors: Structural properties of the biopharmaceutical (e.g., protein sequence), presence of exogenous and endogenous epitopes, degree of glycosylation, exposure of antigenic sites, formulation and storage, down-stream processing, impurities/contaminants, solubility and stability, among others [3,6].
How is immunogenicity measured?
Immunogenicity must be assessed throughout the various phases of clinical development, and although there is typically no immunogenicity monitoring once the drug is on the market a physician may request analysis if a patient stops responding. Although there is a wide variety of assays available, the main types of assays for assessing immunogenicity are:
- Enzyme-linked immunosorbent assays (ELISAs)
- Electrochemiluminescence (ECL) assays
- Radioimmunoprecipitation assays (RIPA)
- Flow cytometry
Regulatory compliance
Immunogenicity evaluation is mandatory for biopharmaceutical regulatory approval and is typically conducted during clinical trials by assessing the presence of ADAs in trial participants [102]. Fully validated, multi-tier assessments are recommended with fit-for-purpose assays included during early clinical trials and non-clinical trials [11]. These assessments ensure that the benefits of the therapeutic offset its potential immunogenicity risk.
Further reading:
For more insights on immunogenicity, browse through the following resources:
- https://www.bioanalysis-zone.com/immunogenicity-considerations-for-aav-gene-therapy-an-interview-with-bonnie-wu/
- https://www.bioanalysis-zone.com/immunogenicity-assessments-for-cell-therapies-an-interview-with-johanna-mora/
- https://www.bioanalysis-zone.com/recent-developments-in-immunogenicity-assays-from-a-cro-perspective/
Watch more on this topic:
- https://www.bioanalysis-zone.com/podcasts/ada-detection-and-characterization-for-immunogenicity-testing-insights-from-icons-lindsay-denhoff/
- https://www.bioanalysis-zone.com/videos/biomarker-and-immunogenicity-testing-an-interview-with-jayaprakash-kotha_worldwide/
- Singlicate ADA analysis – Bioanalysis Zone (bioanalysis-zone.com)
Sources:
- Kuriakose A, Chirmule N and Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. Immunol. Res. (2016) doi: 10.1155/2016/1298473
- Morrow T and Felcone LH. Defining the difference: what makes biologics unique? Healthc. 1(4), 24–29 (2004).
- Pineda C, Castañeda Hernández G, Jacobs IA et al. Assessing the immunogenicity of biopharmaceuticals. 30, 195–206 (2016).
- Tovey MG and Lallemand C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Adv. Drug. Saf. 2(3), 113–128 (2011).
- Walsh G and Walsh E. Biopharmaceutical benchmarks. Biotechnol. 40, 1722–1760 (2022).
- Kessler M, Goldsmith D and Schellekens H. Immunogenicity of biopharmaceuticals. Dial. Transplant. Volume 21(Suppl. 5), V9–V12 (2006).
- Porter S. Human immune response to recombinant human proteins. Pharm. Sci. 90(1), 1–11 (2001).
- Mahanty S, Prigent A and Garraud O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 16, (2015).
- Kropshofer H and Richter WF. Immunogenicity: its impact on ADME of therapeutic biologics. Sciences Encyclopedia. 11, 1–2 (2015).
- Parikh CR, Ponnampalam JK, Seligmann G et al. Impact of immunogenicity on clinical efficacy and toxicity profile of biologic agents used for treatment of inflammatory arthritis in children compared to adults. Adv. Musculoskelet. Dis. (2021).
- Vandivort TC, Horton DB and Johnson SB. Regulatory and strategic considerations for addressing immunogenicity and related responses in biopharmaceutical development programs. Clin. Transl, Sci. 4, 547–555 (2020).
101. US Food and Drug Administration (FDA). Immunogenicity assessment for therapeutic protein products. www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-assessment-therapeutic-protein-products (Accessed 20/07/2024).
102. Brodsky E, Guinn D. Immunogenicity information in human prescription therapeutic protein and select drug product labeling- content and format. www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-information-human-prescription-therapeutic-protein-and-select-drug-product-labeling. (Accessed 20/07/2024).