How does Frontage stay on the cutting edge of bioanalytical advancements?
We interviewed Daniel Sikkema and John Lin (Frontage Laboratories) asking how they stay on top of innovative trends and bioanalytical advancements. They discuss how Frontage ensure quality and what they believe are the critical advancements in identifying new drug targets.
What compelled you to get into bioanalytical research?
Bioanalytical research is an interesting field that is essential to enabling overall public health. Early in our careers, we as junior scientists tend to look up to department leaders, professors, and others with vast experience, so that we can pattern ourselves and learn from their wisdom. This can happen in high school, undergraduate, graduate, or professional training, and the influence of our mentors is often subtle. For myself, this happened initially during my 12 month Medical Technology Internship when I did a rotation with ‘Dr. K’, a retired professor of Biochemistry from University of Michigan (MI, USA) who was heading the ‘Special Chemistry’ Department at the hospital where I did my ASCP internship. During my 4 week rotation in his area, I inquired as to how he decided to pursue a doctorate degree in Biochemistry, and perhaps he recognized something in me during those 4 weeks that caused him to write letters of recommendation for my subsequent application to doctoral programs in scientific disciplines. I became fascinated by the Medical Microbiology field, and I elected to apply for a PhD program at Michigan State University (MI, USA) where I met Professor Robert Brubaker. Professor Brubaker is a bacteriologist studying host-parasite relationships, utilizing Yersinia pestis (causative agent for bubonic plague) as a model for bacterial pathogenesis. Professor Brubaker had previously worked at Fort Detrick in Maryland, and his father had been a senior scientist at Dupont where his work contributed to the discovery of Teflon. I found this fascinating. Knowing my interest in Medical Microbiology, Professor Brubaker recommended an Infectious Diseases post-doctoral fellowship, which then led to a career in immunology, vaccine development, and associated bioanalytical support for immune-mediated fields of medicine in the large Pharma sector. Now, 25 years later, and having had no pre-planned vision for this progression, I still wonder how all these events became synchronized for a kid from a small town in Michigan. Nevertheless, as a seasoned bioanalytical scientist today, perhaps I can be the ‘Dr. K’ for those I manage and interact with going forward, and interest them in the possibilities of performing bioanalytical work to advance public health efforts with modern medicines.
What are the key accomplishments/contributions in bioanalysis and related innovations that directly apply to advancing patient care?
The Frontage team is gratified that many of our sponsors gave us the opportunity to contribute and play a small yet significant role in developing the new medicines available to the patients. Frontage is proud of the research work we do in helping to provide patients with many safe, effective and reliable medicines. Here we just use one example to show how Frontage bioanalytical team made the efforts to support the bioanalysis of the drug products which were approved by US FDA.
DOXIL injection (doxorubicin hydrochloride liposome; Janssen Products, L.P.) is for the treatment of ovarian cancer in patients whose disease has progressed or recurred after platinum-based chemotherapy and for AIDS-related Kaposi’s sarcoma after failure of prior systemic chemotherapy or intolerance to such therapy. A couple of years ago, in response to the critical shortage of Doxil, the U.S. Food and Drug Administration was exercising its enforcement discretion for the temporary importation and distribution of Sun Pharma Global’s Lipodox (doxorubicin hydrochloride liposome injection) in the United States by Sun Pharma Global FZE and its authorized distributor, Caraco Pharmaceutical Laboratories Ltd. Therefore, it was critical for the sponsor to get the product approved by US FDA as soon as possible. However, bioanalysis is one of the challenges in liposomal drug development due to the requirements of separating the free drug and encapsulated drug from human plasma. Also ensuring that there is no drug rupturing during sample processing at the clinic and the bioanalytical lab, during sample shipment, and sample storage. Frontage team successfully overcame these challenges and developed the sample handling procedure and three LC–MS/MS methods for determining the free, encapsulated drug and total drug in human plasma. These methods were successfully applied in the clinical studies. The bioanalytical data were accepted by US FDA and the drug products were approved by FDA.
What do you see as the critical advancements in identifying new drug targets and monitoring disease?
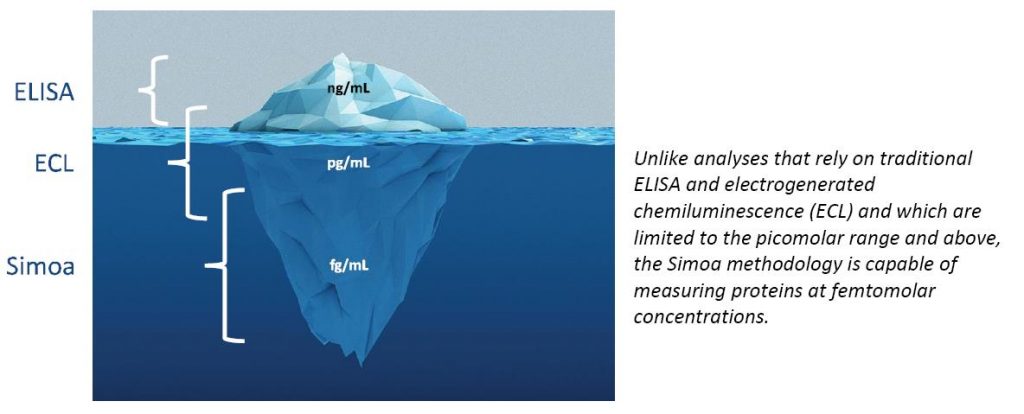
One aspect worth mentioning is the recent advancements in the ultra-sensitive detection of biomarkers, such as the Quanterix Simoa HD-1 Analyzer, which now offers femptogram/mL quantitation of biomarkers (approximately 100–1000 times more sensitive than typical immunoassays). These high-sensitivity detection systems will allow for the identification of many potential new drug targets, as well as the ability to measure an entire host of new biomarkers to monitor drug safety and efficacy.
Not only will this enable new drug discovery and development, but this technology also can be applied to the monitoring of markers of disease remission or progression (Oncology, Infectious Diseases, Neurosciences, Inflammation, Cardiovascular…) using specific protein determinations, rather than using amplified nucleic acids, mammography, CT scan with associated X-ray exposure for head injuries, and other older technologies. Having the ability to accurately and precisely measure these ‘low frequency biomarkers’ using a single molecule analyzer technology opens entirely new pathways for the drug development and patient treatment industry and offers exquisite sensitivity that has never previously been achieved.
Frontage laboratories have invested in the Quanterix Simoa technology and are the first CRO to offer services for GLP and CLIA regulated studies with integration of Watson LIMS to support these ultra-sensitive biomarkers for drug development. Not only ‘off the shelf’ markers are available, but custom antibody pairs used in ELISA methods may be adapted to this platform to improve sensitivity typically 100–1000x or more, giving us the ability to see things that could not be effectively monitored previously. Being able to monitor these markers enables more effective clinical trial design and it is well established that having a suitable biomarker greatly enhances drug development success.
How does Frontage stay on the cutting edge of these innovative trends?
1Trends in LC/MS
#1 LC–HRMS: Frontage bioanalytical team believe that HRMS will play a unique role in the bioanalysis of intact molecule quantification and endogenous analytes, etc. Frontage invested in state-of-the-art equipment and demonstrated the capabilities of using HRMS to address the bioanalytical challenges related to the ADC bioanalysis (i.e. DAR determination) and the specific form of the protein biomarker in plasma. HRMS in conjunction with liquid chromatography (LC–HRMS) has become available to many laboratories in the pharmaceutical industry. Due to its enhancedspecificity using the high resolution power and its capability of simultaneous quantitation and structural elucidation using the post-data acquisition data mining feature, utilization of LC–HRMS for bioanalysis could lead to potential rapid and reliable method development as well as sample analysis; thus generating both cost and resource savings. We believe when utilized appropriately, LC–HRMS will play a significant role in the future landscape of quantitative bioanalysis.
#2 Large molecule quantification using LC–MS: LBAs are widely used for bioanalysis of large molecules, but these assays can be lengthy to develop and have limited selectivity and sensitivity. LC–MS–MS-based bioanalysis can offer important advantages over immunoassay-based techniques, but LC–MS–MS with large molecules introduces several challenges affecting sensitivity, selectivity, and data analysis. Frontage has a dedicated team supporting large molecule bioanalysis using LC–MS and has developed many methods with the sensitivity and selectivity to support the PK studies of ADC, antibody and protein drug candidates. We can forecast more applications of LC–MS in large molecule bioanalysis due to its advantage on selectivity and multiplexing capability.
#3 Microsampling: In recent years there has been much discussion related to the use of fewer animals for pre-clinical safety and efficacy testing which helps advance our commitment to the 3Rs—replace animal models where possible, reduce the number needed, and refine our processes to get the data required with minimal use of animals and microsampling in a pediatric setting due to the limited sample volume available for bioanalysis. Recently a Capillary Microsampling Technique (CMS) has been developed wherein a flexible plastic capillary tube, lined with K2EDTA, is used for the collection of 35–40 µL of blood by Frontage team. This tube is then spun down and approximately 20–25 µL of plasma is available. Small metered sections of the plasma section of the capillary are then cut into multiple sections and stored for the initial analysis, re-assay, and ISR. Each section of the capillary is placed into a secondary container, diluted with a washout solution and extracted using protein precipitation. The container is then spun down, the supernatant further diluted with mobile phase, and analyzed using LC–MS/MS. In mouse plasma, this method was found to be accurate and precise in a curve range of 5–2000 ng/mL. With this method a complete PK curve can be determined from a single mouse thereby reducing the number of animals in each study. More importantly, in pediatric models, a heal, or finger, stick can be used to extract very small volumes of blood for testing. The advantages of Frontage CMS methodology include use of plastic vs. glass tubes, multiple samples available for initial sample analysis, reanalysis, and ISR test, avoid issues related to sample timing; 5 min, 15 min, 30 min, etc., avoid the sample dilutions on site vs. other CMS method with sample dilution, avoid possible BQL samples due to dilution, application in the pediatric studies.
#4: Paperless Lab: Regulated bioanalytical laboratories generate enormous amounts of data for regulatory submission and approval. Paper-based or hybrid lifecycle data management in the bioanalytical laboratories is common even though it has been recognized as inefficient, and includes potential compliance risks in the drug development process. Paperless processes based on standardized procedures (defined business rules), real-time acquisition/storage, and access to data will be the trend for the bioanalytical laboratory operation. Frontage has implemented a few critical data management components such Watson LIMS, ELN, etc and in the processing converting the paper-based data management system to the electronic data management system (paperless lab). As data management systems move beyond formal forms-driven processes to include a true closed loop design to manage disparate processes across the enterprise, they can provide support for collaborative processes and deliver insight into the overall state of control with the potential to close the gap between simply accomplishing regulatory compliance and delivering measurable improvements in quality and efficiency.
2Trends in biologics
Frontage is a full-service CRO provider and invests heavily in the latest technology to support an increasingly complex drug development portfolio (see 3 and 4a above). By keeping abreast of the latest technology, participating in key workshops and drug development symposia, Frontage ensures that we maintain the most advanced and differentiating skills to support past, current, and future drug development scenarios. Further developments are rapidly taking place in the Immuno-Oncology space, and also the Cell/Gene Therapy space. We are entering an age of personalized and precision medicine, as well as regenerative medicine, and the opportunity to support these new advancements is particularly exciting from both a scientific and a public health perspective. Frontage investments in technology and scientific personnel ensure that we are well equipped to support these rapidly developing fields of medicine enabled by new drug modalities as they continue to evolve. Having the appropriate analytics to support these new modalities is a constant challenge to the industry and requires leadership that recognizes and embraces these challenges through investment in cutting edge technologies.
3Trends in biomarkers
As with any technological field, having a suite of analytical tools allows us to take a technologically agnostic approach to supporting a given analytical challenge, and turning it into a solution. Whether it be sample volume, assay cost, assay sensitivity, discrimination of isoforms, etc., a comprehensive suite of available technologies allows us to recommend and implement several scenarios to meet the needs of the program. Also, participating in workshops, such as the AAPS and US FDA Crystal City VI Workshop on Bioanalytical Method Validation for Biomarkers held on September 28–29, 2015, ensures that we apply the latest regulatory understanding and industry best practices to our technology to enable these new medicines to reach the patients that will benefit from them.
Onco-Immune, Rare diseases, Infectious Diseases, Neurosciences, Respiratory, Cardiovascular, Inflammation, and other drug development programs all benefit significantly when an appropriate biomarker is available. This can greatly reduce the overall cost for clinical development by stratification of patients, early detection of drug/benefit signals, early detection of adverse events, and the potential for diagnostic development.
In the ever-advancing field of bioanalysis, how do you continue to ensure quality and adapt your processes to ensure compliance?
Frontage closely monitors the changes in the regulations from various regulatory bodies to ensure that our bioanalytical data in the submission meeting the compliance requirements of US FDA and EMA, but also other regional regulatory agencies such as cFDA (China), MHLW (UK), Japan, Health Canada, etc.. We maintain involvement with organizations like Society of Quality Assurance and subsidiaries Bioanalytical subsection (BASS) and Mid-Atlantic Regional SQA, attend meetings such as WIRB, and EBF, attend Crystal City meetings, and are involved with the Global CRO Consortium. We actively participate in surveys about how requirements are being met. We keep abreast of literature and have also published articles and chapters. And, while we have a good experienced group of staff, we gain additional perspectives by interactions with our clients and with regulators.