Merck: BioSPME microsampling

Website: http://sigmaaldrich.com/biospme
Contact: [email protected]
Profile: There is growing interest in clinical and bioanalytical research fields toward microextraction or microsampling techniques for sample isolation, storage, and transportation. This article describes an innovative, 1-step, rapid microsampling and sample prep technique that is both selective and quantitative, providing many advantages over dried blood spots and other microsampling technologies that currently exist. This technique is simple and has endless applications, including those for LC/MS, LC/MS/MS, and Direct MS. For more information, visit sigmaaldrich.com/biospme
View this webinar from GSK to learn more about the benefits of this technology:
Solid Phase Microextraction: Sketching the Future of Microsampling in Bioanalysis – GSK
The Advent of Bioanalytical Microsampling using Biocompatible SPME and Benefits over Dried Blood Spots
There is growing interest in clinical and bioanalytical research fields toward microextraction or microsampling techniques for sample isolation, storage, and transportation. This interest comes from cost and ethics standpoints as researchers seek an inexpensive alternative to terminal blood draws. In this article we will present solid phase microextraction (SPME) as a viable technique for microsampling of biological matrices for subsequent HPLC, UHPLC, and LC/MS analysis. The use of SPME in bioanalytical applications has been the topic of recent publications.1 The fibers allow direct sampling of biological matrices without additional sample treatment. The concentration effect of the fibers provides increased analyte sensitivity compared to other techniques.
Biocompatible SPME Fibers: Design and Function
SPME for bioanalytical microsampling (BioSPME) comprises particles (e.g. functionalized silica) embedded in an inert binder and immobilized onto durable, flexible metal fibers. The coated fibers are then inserted into hypodermic needles (SPME LC Fiber Needle Probes) or pipette tips (SPME LC Tips) for in vivo, ex vivo, and in vitro microsampling applications. Because they are compatible with solvents routinely used in liquid chromatography (LC), the fibers are called SPME LC. This terminology distinguishes them from the traditional SPME fibers used with gas chromatographic analysis. In principle, the particles can be modified with any LC-type stationary phase, but this study utilized particles with the popular C18 chemistry. The binder excludes matrix-derived large biomolecules, while allowing small molecules to penetrate and be adsorbed onto the embedded particles. Extraction is based on an equilibrium process in which the analyte partitions between the SPME fiber coating and the sample matrix (Figure 1.)
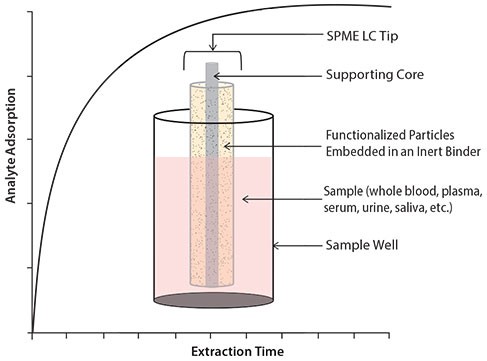
The adsorption mechanism for SPME is based on partitioning of analytes between the solution (sample) and the fiber coating. The rate of this partitioning is dependent upon the affinity of the analyte for the phase coating compared to the affinity for the sample matrix. SPME is not an exhaustive technique. After a given amount of time, equilibrium is achieved between the concentration of analytes in the sample matrix and the fiber coating.
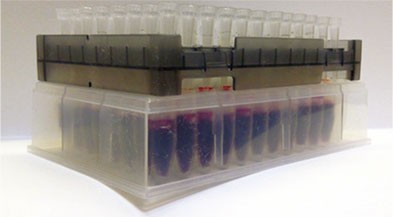
BioSPME is designed for the direct sampling of biological matrices, eliminating the need for protein precipitation, centrifugation, and sample freezing/thawing. The entire BioSPME approach, whether using the SPME LC Needle Fiber Probes or SPME LC Tips, is unique in that sample concentration and sample cleanup are conducted simultaneously, thereby reducing the overall number of sample processing steps. There is no sample volume requirement, minimum or maximum, in BioSPME. The volume needs only be enough to cover the fiber and can be very small depending on the conformation of the sample vessel. The SPME LC Tips in 96-tip array are amenable to robotic handling and automation (Figure 2).
Aim of Current Study: Comparison of BioSPME to DBS
This study compares BioSPME using SPME LC Tips to dried blood spots (DBS) in terms of UHPLC/MS response (extraction efficiencies and detection limits) of target analytes from whole blood using a set of model compounds. The chromatographic conditions appear in Figure 3.
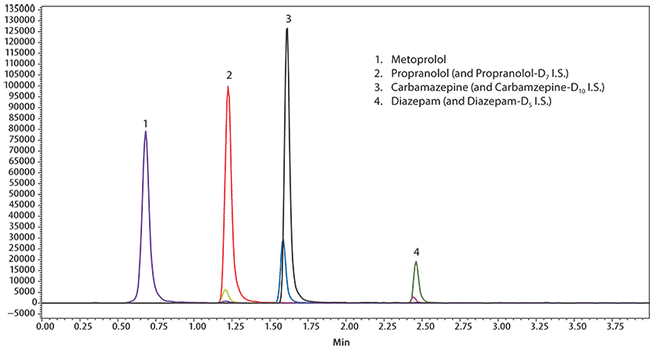
Figure 3. UHPLC/MS Analysis of Metoprolol, Propranolol, Diazepam, and Carbamazepine in Whole Blood on Titan C18 after Solid Phase Microextraction (SPME) using Biocompatible C18 Fibers water was removed by capillary action using filter paper. The tips were desorbed for 30 minutes in a conical HPLC vial with 50 μL of methanol containing 20 ng/mL of internal standard. Desorption was conducted on an IKA MS 3 shaker for 30 minutes at 500 rpm. Samples were then analyzed directly by LC/MS/MS.
Experimental
Metoprolol, propranolol, carbamazepine, diazepam, and their deuterated analogs were acquired from Cerilliant. Stock solutions of 10 ng/mL of each component were used for preparation of calibration solutions along with spiking blood samples. A combined stock solution of deuterated internal standards was prepared at 20 ng/mL. Human whole blood samples with hematocrit (HCT) levels of 45% were acquired from BioreclamationIVT (Baltimore, MD, USA). Whole blood samples were spiked at 50 ng/mL total analyte concentration and equilibrated for one hour. A 300 μL aliquot of each sample was added into a 96-well conical plate (E&K Scientific Products, Inc., Santa Clara, CA, USA) for extraction.
BioSPME Extraction
Figure 4 depicts the extraction process of whole blood using the SPME LC Tips in the 96-tip configuration. The tips were preconditioned in acetonitrile:water (50:50) for 20 minutes prior to extraction, exposed to the sample for 15 minutes with vortex agitation at 500 rpm on an IKA® MS 3 shaker, removed from the samples, and washed in distilled water for 30 seconds. Excess water was removed by capillary action using filter paper. The tips were desorbed for 30 minutes in a conical HPLC vial with 50 μL of methanol containing 20 ng/mL of internal standard. Desorption was conducted on an IKA MS 3 shaker for 30 minutes at 500 rpm. Samples were then analyzed directly by LC/MS/MS.
Dried Blood Spot Media
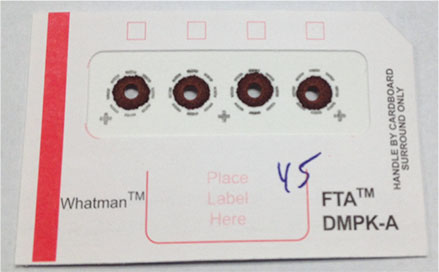
Figure 5 shows the DBS card spotted with whole blood after removal of the 3 mm punch. A 20 μL aliquot of the sample was pipetted onto the Whatman™ FTA™ DMPK-A DBS card (GE Healthcare, Piscataway, NJ, USA), which was then allowed to dry overnight. A 3 mm punch from each spot was placed into a 100 μL conical vial and desorbed with 50 μL of methanol containing 20 ng/mL of internal standard for two hours. The remaining punch was removed from the vial prior to LC/MS/MS analysis.
Comparison of Analyte Recovery
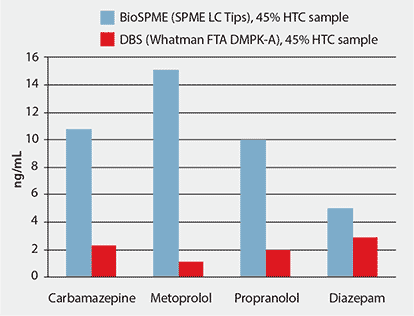
The absolute amount of desorbed analyte from the DBS card was much lower than the BioSPME method using SPME LC Tips.
Figure 6 shows the higher detected analyte concentration on the SPME LC Tips compared to the DBS card. This improvement in response from BioSPME comes primarily from the concentrating ability of the fiber coating, but also from the small desorption volumes needed to displace the analytes from the fibers. In contrast, the relatively small portion of sample obtained from the 3 mm punch of the DBS card and the large desorption volume required to fully cover the punch, limit the amount of sample that can be detected by the analytical method without evaporation to concentrate the sample. The result is analyte detection levels that are five- to ten-times higher using the SPME LC Tips compared to the DBS card.
Conclusion
BioSPME, whether using the SPME LC Needle Fiber Probes or the SPME LC Tips described here, is a convenient and selective microsampling technique and a viable alternative to DBS cards for isolation of small molecules from biological matrices such as whole blood, plasma, serum, urine, and saliva. BioSPME in the pipette tip format allows for single sample or batch processing, and can be automated with various liquid handling systems. Manual use of BioSPME tips can be performed by transferring all 96 tips within the tray holder, allowing for simplified handling should a liquid handler not be available. In addition to concentrating the target analytes, the fiber coating shields sample matrix from being absorbed onto the fiber, thus allowing for cleaner samples. Previous studies have demonstrated increased analyte levels with a significant reduction in sample matrix using BioSPME technique compared to traditional protein precipitation approaches.