Poster: Enhancing the sensitivity of a hybridization-assay for the measurement of an antisense oligonucleotide in clinical samples
Abstract
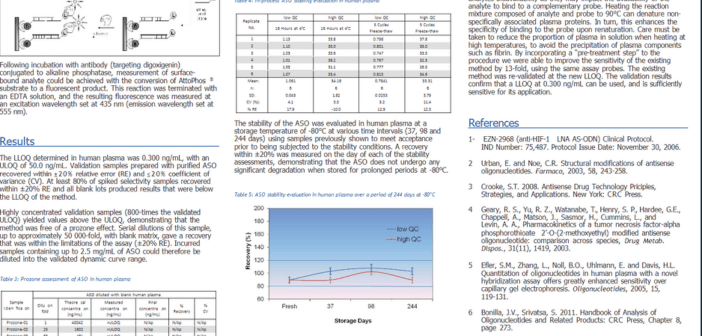
Purpose: Although an existing partially validated PK method existed for the measurement of an Antisense Oligonucleotide (ASO) in human plasma [lower limit of quantitation (LLOQ) of 4.00 ng/mL], it was not sufficiently sensitive for an upcoming clinical trial (Phase II). The purpose of this study was to optimize and fully re-validate a hybridization-assay for its intended application.
To read more, download the full poster below.
This article is part of the Bioanalysis Zone Spotlight on the practical challenges of new modalities in bioanalysis. For more expert opinions on this topic, visit our feature homepage
In association with:

