Non-invasive identification of circulating tumor cells in the bloodstream
A joint research team from Tufts University and Northeastern University (both MA, USA) have developed a novel technique for the detection of circulating tumor cells, which will facilitate early diagnosis of cancer metastasis.
Cancer cells that have metastasized and spread throughout the human body tend to complicate cancer treatments, therefore the early detection of cancer metastasis is essential. Circulating tumor cells (CTCs) in the blood or lymph vessels are a common biomarker used for the detection of cancer, however, their presence can often be rare or non-existent in small blood samples, despite appearing in the patient’s bloodstream.
Diffuse in vivo flow cytometry (DiFC) is a newly established technique helping to solve this issue. The process requires tagging CTCs with a fluorescent agent by directing a laser straight onto an artery. A detector is then used to capture the emitted fluorescent signals and quantify the number of CTCs. Although showing promise, DiFC measurements can be significantly influenced by background noise, which is caused by the inherent fluorescence of surrounding tissues, known as autofluorescence.
The cooperative research team from Tufts University and Northeastern University have been actively addressing this issue and improving upon the DiFC method. The team employed the dual-ratio approach as a means of minimizing noise in DiFC. The dual-ratio approach combined with DiFC uses two laser sources and two detectors, rather than just one, helping to cancel out the unwanted noise and autofluorescence contributions of the measured surface.
In their latest study, published in the Journal of Biomedical Optics, the researchers ran three main experiments to test the advantage of combining the dual-ratio approach and DiFC. Monte-Carlo simulations were conducted using several noise and autofluorescence parameters, alongside various source-detector configurations. Dual-ratio DiFC experiments were performed with an artificial tissue-mimicking flow phantom with cell-mimicking fluorescent microspheres. To conclude, the autofluorescence of the skin was also measured to understand the variation of noise with tissue depth and type.
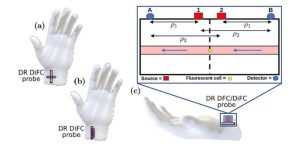
The dual-ratio diffuse in vivo flow cytometry (DiFC) is a novel technique that uses two laser sources and two detectors to mitigate the effects of noise and autofluorescence, which may enable doctors to precisely detect fluorescent tags attached to circulating tumor cells in the bloodstream. Credit: Blaney et al., 10.1117/1.JBO.28.7.077001
The results of the experiments revealed that dual-ratio DiFC was superior to standard DiFC. Autofluorescence is typically higher in skin compared to the underlying muscle, implying that dual-ratio DiFC may prove advantageous over standard DiFC in most cases.
The results of this study are crucial in advancing the application of dual-ratio DiFC as an emerging, non-invasive method for detecting fluorescent molecules in the bloodstream. Implementation of this technique will allow medical professionals to rapidly identify cancer cells in patients’ blood without the need for invasive sample collection. Additionally, this approach holds the potential for extension to other cell types or even systemic molecules of interest in future experiments.





