New microfluidics system can separate different cell types within a microdroplet
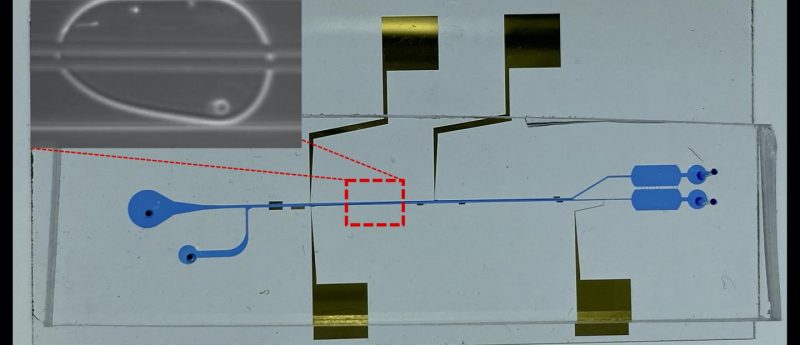
A research team from Texas A&M University (TX, USA) has developed a new high-throughput method for cell separation, that can be used in combination with droplet microfluidics.
The team used an electric field to successfully isolate pathogens attached to host cells from pathogens that were unattached within a single fluid droplet. The findings were reported in the journal Lab on a Chip.
“Other than cell separation, most biochemical assays have been successfully converted into droplet microfluidic systems that allow high-throughput testing,” explained Arum Han, Texas A&M University (TX, USA).
“We have addressed that gap, and now cell separation can be done in a high-throughput manner within the droplet microfluidic platform. This new system certainly simplifies studying host-pathogen interactions, but it is also very useful for environmental microbiology or drug screening applications.”
A microfluidic device is made up of micron-sized channels that allow for regulated movements of fluids. Microfluidics using water-in-oil droplets have recently become more popular for a various biotechnology applications. The droplets are picolitres in volume and can be used for transporting biological materials or carrying out reactions. A chip containing millions of droplets could enable high-throughput experiments that would save laboratory space and labour and reduce the cost of chemical reagents.
You may also be interested in:
- Portable testing device clipped to smartphone could detect pathogens in minutes
- Five recently developed microfluidic devices
- The age of lab-on-a-chip technology is now – an interview with Aaron Wheeler
The research team selected a host-pathogen model system comprising of the human macrophage and salmonella bacteria, to develop the cell separation technology. The aim of the experiment was to separate the salmonella that was attached to the human macrophage from the salmonella that was not attached.
“Getting cell separation within a tiny droplet is extremely difficult because, if you think about it, first, it’s a tiny 100-micron diameter droplet, and second, within this extremely tiny droplet, multiple cell types are all mixed together,” Han continued.
The research team structured two pairs of electrodes that produced an oscillating electric field near the droplet host-pathogen model. The force of the electric field experienced by the cells differed- due to the different properties of the host cell and pathogen cell. The electric field caused the cells to move at different times, resulting in the cells being divided into two separate locations within the droplet. A downstream Y-shaped splitting junction was used to separate the mother droplet into two daughter droplets containing one type of cell.
The experiments were conducted with a host-pathogen model whose interactions are well-established. However, the new microfluidic system equipped with droplet separation is most beneficial when the pathogenicity of bacterial species is unknown.
The technology allows for quick, high-throughput screening where cell separation is required.
“Liquid handling robotic hands can conduct millions of assays but are extremely costly. Droplet microfluidics can do the same in millions of droplets, much faster and much cheaper. We have now integrated cell separation technology into droplet microfluidic systems, allowing the precise manipulation of cells in droplets in a high-throughput manner, which was not possible before,” Han concluded.
Sources: Han SI, Huang C, Han A. In-droplet cell separation based on bipolar dielectrophoretic response to facilitate cellular droplet assays. Lab chip. 20, 3832–3841 (2020); https://today.tamu.edu/2020/11/30/microfluidic-system-with-cell-separating-powers-may-unravel-how-novel-pathogens-attack/
Feature image: An image of the in-droplet cell separation microfluidic chip, showing the microfluidic channels and electrodes. Enlarged view shows a host cell and pathogenic bacteria cells being separated to top and bottom within a single water-in-oil microdroplet. Credit: Dr Arum Han/Texas A&M University College of Engineering






