It’s about how we react

In this column from Robert MacNeill (Pharmaron; PA, USA), he discusses the transition from mass spectrometry to tandem mass spectrometry and the relationship between the ‘parent’ ion and the ‘daughter’ product.
 Robert MacNeill received his Bachelor’s degree with Honors in Chemistry from Heriot Watt University and then his MSc in Analytical Chemistry from the University of Huddersfield, both in the United Kingdom. Robert is also a Chartered Chemist and a Fellow of the Royal Society of Chemistry. With 20 years of experience in all aspects of quantitative bioanalytical LC–MS/MS method development, 11 of these years heading method development activities within HLS/Covance, and a regular author and peer reviewer for the journal Bioanalysis, Robert is a recognized expert and innovator in the field.
Robert MacNeill received his Bachelor’s degree with Honors in Chemistry from Heriot Watt University and then his MSc in Analytical Chemistry from the University of Huddersfield, both in the United Kingdom. Robert is also a Chartered Chemist and a Fellow of the Royal Society of Chemistry. With 20 years of experience in all aspects of quantitative bioanalytical LC–MS/MS method development, 11 of these years heading method development activities within HLS/Covance, and a regular author and peer reviewer for the journal Bioanalysis, Robert is a recognized expert and innovator in the field.
In his current role, Robert coordinates all LC–MS/MS method development and associated training, takes the lead in keeping abreast of innovation and technological development in the industry, leads in-house research projects and performs technical writing for the purpose of producing publications.
Amid the upbeat conversational bustle of the animated crowd assembled in the graduation hall, Dr Molly Cuellar beamed at her gowned and decorated companion.
“You are my daughter, Iona. I am the proudest parent. Yes, there have been times when I felt fragmented and we’ve had our pressurized collisions, but we harnessed that energy in the right way and you have learned to be specific and focused, sweeping aside all interferences from the clarity of your way forward. But, somehow, I still feel as structured as I have always been.”
“I know I’m finding my stable path, Mom! Thank you for being there as a precursor to everything I’m involved in! We may need our offsets and our breaks, but together we make something special, regardless of whether it’s a nominal matter or one demanding of a higher resolution.”
Transitioning from mass spectrometry to tandem mass spectrometry, as it is known, is a leap onto a splendid new plane of offerings. Tandem mass spectrometry is indeed long established as the bearer of profound new levels of specificity, sensitivity and applicability to an assay. The possibility of multiple stages, also tandem in space and tandem in time depending on your available toolkit and technology, only serves to propagate the interest factor and useability. It must have been wonderful to be first struck by the essence of this idea and suffice to say, something useful certainly emerged from it.
In the fundamental process of tandem mass spectrometry in a fully quantitative workflow, the sequence is as follows. A precursor (parent) ion is filtered from potential interferences of all other m/z through one mass spectral analyzer unit, purposefully fragmented in a pressurized cell and then the product (daughter) ion is filtered through a final analyzer. In a traditional triple quadrupole instrument, by far the most familiar to GLP quantitative bioanalysis, zero-width monitoring is applied in each key quadrupole, referred to as single and multiple reaction monitoring. Zero width means there is no sweeping of a range of m/z, in other words, the instrument’s resolution setting applies in both relevant quadrupoles, usually ‘unit’ which corresponds to 0.7 FWHM (full width at half maximum) of the mass spectral peak. The resolution may be altered to benefit a methodology in the face of certain challenges, but that is another article in itself. The area of collisional activation is another quadrupole but set to ‘RF-only’ essentially transmitting ionic abundance of all m/z present. There is a collision offset, frequently referred to as ‘collision energy’ a potential difference between the entrance and exit lenses accelerating the ions through the quadrupole, and this is key, alongside the gas pressure setting, in the product ion formation. The product ion is identified within the aptly named product ion scan, where only the precursor ion is transmitted into the collision cell and the relevant parameters of collision gas pressure and collision offset are optimized as a crucial part of the instrumental ‘tuning’ when constructing the method. Altogether, the outcome and bottom line is a method with terrific sensitivity and selectivity, with baselines vastly reduced and stable, in comparison to the predicament of single quadrupole operation, otherwise commonly known as ‘MS-only.’
Tandem mass spectrometry is also frequently denoted by MS/MS with the forward slash indicative of one stage of fragmentation involved. There are all kinds of scans one may perform in the realm of MS/MS, with more qualitative outcomes in mind. Precursor, neutral loss, product, Q1 and Q3 scans, all making use of the fundamental operation and all involving a sweep of a specified range of m/z.
Then there are ion trap instruments, bringing other ways of using fragmentation. There are traditional traps that confine ions to a point in space, sometimes referred to as 3D-Traps, which have great features such as the capability to perform MSn, multiple sequential fragmentations. However, they suffer from what is known as space-charge effects, which occur due to the very nature of the proximity of the ions in their trajectories, taking away from sensitivity and resolution and a low mass cut-off for each fragmentation. The traditional ion trap represents the prime example of ‘tandem in time.’ There are linear ion traps, based on a triple quadrupole mass filter rail, which effectively overcomes the space-charge limitation and still offers MS/MS/MS (MS3) overall, not including any desired cone voltage fragmentation. The trap product ion scanning is very sensitive and can reach in excess of 10,000 resolution when using slow scan speeds, showing far reduced space-charge effects and freedom from a low mass cut-off. However, with MS3, the final fragmentation is in the same quadrupole as the preceding product ion’s isolation and gives a low mass cut-off. In any case, there is a modest variety of useful scan modes and, on the whole, this kind of instrument is synonymous with quantitative application and may be said to offer both tandem in-space and tandem in-time operations.
Lastly, the breakpoint, in that the final analyzer need not be of a nominal mass nature, where nominal pertains to quadrupolar detection and the approximate 4000 resolution it offers. The contemporary outlook is of accurate mass detection gaining a foothold in regular qualitative and quantitative application. The reasons, embedded in the high-resolution nature of these technologies, are entirely convincing. The benefits of high-resolution speak for themselves, especially for complex biologics and the analytical challenges such as those presented by antibody–drug conjugates, where we might well strive for the ideal of all components to be analyzed on the same platform, but again this is material for another article. Examples are quadrupole time-of-flight (QTOF) technology, offering a resolution of approximately 50,000, which marries splendidly with fast chromatography and also gives impressively high-sensitivity methods in latest-generation models. With such technology, it is easy to use TOF-MS, no fragmentation, to give accurate precursor ion measurement and confirmation ready for translation to tandem MS workflows. There is also Orbitrap technology, in which commercial manifestations can strike a challenge with the order of several hundred thousand resolution.
As activating as these ideas and realities are, at this point, we must break it off and conclude. In bioanalytical life nowadays, to my awareness, it very seldom happens that an acquisition of a single-stage instrument is made in preference to a tandem. Sensitivity and selectivity are in such incredible, ubiquitous demand that it’s a necessity. The question has far more to do with the selection of the scanning nature of the final analyzer. Mainly, it is down to the choice of nominal mass or accurate mass. Both have their place, but the latter is, in the big picture, set to dominate the future. Either way, this is where ‘picking up the pieces’ is seen in a very beneficial context.
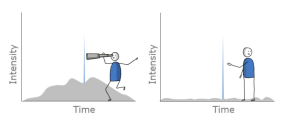
Sincere thanks to Hazel Dickson from Waters Corporation (MA, USA) for the visual.
Disclaimer: the opinions expressed are solely my own and do not express the views or opinions of my employer.
Our expert opinion collection provides you with in-depth articles written by authors from across the field of bioanalysis. Our expert opinions are perfect for those wanting a comprehensive, written review of a topic or looking for perspective pieces from our regular contributors.
See an article that catches your eye? Read any of our articles below for free.