First satellite bioanalytical lab offers fastest option for PBMC processing in Australian clinical trials

In this article, Agilex Biolabs (Adelaide, Australia) announces its establishment of its first satellite processing unit staffed with specialized technicians on the fifth floor of the CMAX Clinical Research facility. This collaboration could see improved sample processing time and more reliable results within the bioanalytical field.
On 09 September 2022, Australia welcomed its first-ever bioanalytical laboratory inside a Phase 1 clinical unit.
Agilex Biolabs, the leading bioanalytical laboratory supporting Australian clinical trials, has established its first satellite processing unit staffed with highly specialized technicians on the fifth floor of the CMAX Clinical Research facility in Adelaide, SA. The Agilex satellite laboratory will perform time-sensitive sample processing techniques in the same building as clinical trial participants, opening the door for new drug sponsors to reduce risk in their studies by truncating the decline of sample integrity.

Peripheral blood mononuclear cells (PBMCs) include several hallmark cell types involved in immune response, such as T cells, B cells, and NK (natural killer) cells. The count of a certain blood cell type is often a critical piece in assessing the impact of a drug on the immune system. Similarly, cytokines released by immune cells can indicate the level of immune response or unwarranted inflammatory response, such as cytokine storm.
As soon as a blood sample is drawn from a person’s body, that sample begins to deteriorate as blood cells are no longer resupplied with oxygen. When a clinical trial hinges on a reliable count of PBMCs or cytokines, whole blood from the patient must be processed as quickly as possible to stop deterioration and ensure that quantitative analysis of the sample is an accurate reflection of the cells in the human they came from. The sooner a whole blood sample is treated, the less deterioration of cells has occurred and therefore sample integrity is higher.
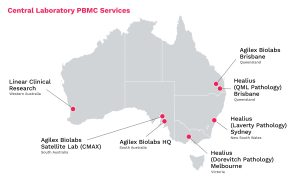
While clinical sites are well-equipped to draw samples from clinical trial participants, they are not normally outfitted with a specialized laboratory where sample preparation can take place. Instead, after a sample is taken from the vein of a patient it must immediately be transported by truck to another location for processing. Through the Healius network and Linear Clinical Research, offsite PBMC processing is accessible to Phase 1 units throughout Australia.
“Even when the processing lab is only a few kilometers from the clinical site, risk accumulates with every passing minute,” said the CEO of CMAX, Jane Kelly. “An extra stoplight here, a road closer there—the integrity of a sample is declining, so every trip is a race against time. In partnership with Agilex, we are eliminating that risk from CMAX clinical trials.”
At CMAX, a blood sample will soon go from patient to processing at a specialized laboratory unit in 15 minutes—the Agilex satellite laboratory is only meters away.
Technicians at the Agilex laboratory on the 5th floor can immediately stop the clock and begin processing samples. In addition to isolating PBMCs from whole blood, Agilex laboratory specialists are also trained in other time-sensitive sample preparation techniques, such as stimulation of whole blood for cytokine release assays or stimulation of isolated PBMCs for studies measuring NLRP3 inflammasomes.
Following onsite processing, samples are shipped quickly to the nearby Agilex Biolabs headquarters also located in Adelaide. With their rapid-turnaround sample analysis team, Agilex provides reliable quantitative data within just a few days of sample collection at CMAX.
PBMC quantitation by flow cytometry is just one of many bioanalytical services Agilex performs for biopharma clients worldwide—the sprawling campus includes multiple facilities for large and small molecule bioanalysis, supporting new and repurposed drug programs in many therapeutic areas including immunology, immuno-oncology, infectious diseases, vaccines, and many others.
In a steadfast commitment to quality and speed, the satellite laboratory is just one of several leaps in innovation Agilex is making to improve pipeline advancement for their customers.
“This is a huge win for clinical trials in Australia,” said Agilex Chief Scientific Officer, Kurt Sales. “Until now, time-critical whole blood stimulation or PBMC processing had to occur at off-site processing units in Adelaide. A clinical research facility with onsite processing capabilities presents an opportunity to achieve a faster turnaround of sample processing and more reliable results.”
This collaboration between Agilex and CMAX improves the reliability of bioanalytical data by conserving sample integrity with faster processing. Equipped with the services of Agilex and CMAX, drug sponsors can have confidence in their data and save time getting through clinical trials to deliver new, safe, and efficacious therapies to patients with unmet needs.
Agilex Biolabs is Australia’s largest and most technologically advanced regulated bioanalytical and toxicology laboratory. For 25 years, Agilex has equipped biopharma companies around the globe with reliable and defendable, clinical bioanalysis, biomarker data and toxicology studies as they champion new therapeutics and modalities that improve human health. Our global clientele includes drug sponsors shepherding small molecules, biologics, cell and gene therapies, and vaccines through preclinical and clinical development. Agilex is a proud member of the Healius network.
One of Australia’s leading healthcare companies, Healius is synonymous with quality, affordable and accessible healthcare for all Australians. It has an expansive network of pathology laboratories, diagnostic imaging centers and day hospitals. Healius provides quality healthcare services that are easily accessible and cost-efficient while supporting the coordination and continuity of quality patient care.
CMAX Clinical Research has been a leader in delivering early-phase clinical trials for over 28 years, making it one of the most respected clinical trial businesses in Australia. Since 1993 CMAX has delivered more than 700 early-phase clinical trials, including more than 150 first-in human studies. CMAX’s modern facility is equipped with 78 inpatient beds, inclusive of a new low-stimuli suite and negative air-pressure isolation rooms, and has ready access to state-of-the-art facilities, equipment, and world-class medical and pharmacology specialists.
In association with: