SVAR Life Science

Svar is a GxP-certified bioanalytical CRO focusing on various bioassay services – both ligand binding and cell-based. Svar has more than 25 years long experience in laboratory services, bioassay development and in-house manufacturing of products and components.
With the unique paring of services and products Svar Life Science has become an ideal partner for many pharma and biotech companies. Today, it offers GLP/GCP Bioanalytical Services and GMP QC Testing Services specifically focusing on large molecules.
Svar distinguishes itself by offering unique technology platforms, including iLite cell-based solutions, complement system solutions and calprotectin solutions. All the platforms have been developed by experienced scientists in close collaboration with key opinion leaders.
Our range of Bioanalytical Services includes assay development, validation and sample analysis for pre-clinical and clinical studies. We have experience with a wide range of immunoassays, including ELISA, MSD, SIMOA and cell-based assays.
Our GCP, GLP, GMP and ISO 15189-compliant laboratory is managed and staffed by scientists with significant experience in regulated environments and bioassay development.
Being a customer-focused organization, Svar Life Science always strives to meet the individual needs and requirements of each customer.
Service areas and areas of focus:
- PK
- TK
- Immunogenicity
- Biomarkers
- PD
- Potency
- Consultancy services
- Bioassay development
- Bioassay qualification validation
- Lot release potency
- Stability testing
- In-process testing
Stay up to date on our latest news! Read the Svar Life Science blog here
 In this Editorial Article, Dr. Michael Tovey, Chief Scientific Advisor & inventor of the iLite® technology at Svar Life Science, highlights how cell-based assays are helping the development of complex biotherapeutics. The article also presents how this advanced engineering platform works and how it enables the design and construction of complex and sophisticated components. The possibilities are endless when it comes to the potential number of target combinations.
In this Editorial Article, Dr. Michael Tovey, Chief Scientific Advisor & inventor of the iLite® technology at Svar Life Science, highlights how cell-based assays are helping the development of complex biotherapeutics. The article also presents how this advanced engineering platform works and how it enables the design and construction of complex and sophisticated components. The possibilities are endless when it comes to the potential number of target combinations.
 In this Editorial Article, Dr. Peter Betz Wolff, of the Analytical Development department at AGC Biologics, who works to implement and develop a range of cell-based assays to meet specific client needs, shares how his team recommends the use of assay-ready cells to provide the valuable biologically relevant data required for robust pharmaceutical development.
In this Editorial Article, Dr. Peter Betz Wolff, of the Analytical Development department at AGC Biologics, who works to implement and develop a range of cell-based assays to meet specific client needs, shares how his team recommends the use of assay-ready cells to provide the valuable biologically relevant data required for robust pharmaceutical development.
Complement in drug discovery – The key to addressing a large unmet therapeutic need
 In this Editorial Article, Előd Körtvély, principal scientist at Pharma Research and Early Development (pRED), Roche Innovation Center, Basel, shares how the team in Ophthalmology strives to develop novel therapeutic approaches for major eye diseases.
In this Editorial Article, Előd Körtvély, principal scientist at Pharma Research and Early Development (pRED), Roche Innovation Center, Basel, shares how the team in Ophthalmology strives to develop novel therapeutic approaches for major eye diseases.
The article also deals with how the drug discovery process within the complement field has evolved and what the key challenges in this field are.
 In this editorial article, Dr Michael Tovey, Chief Scientific Advisor & inventor of the iLite® technology at Svar Life Science, highlights how cell-based assays are helping the development of complex biotherapeutics. The article also presents how this advanced engineering platform works…Read more & download
In this editorial article, Dr Michael Tovey, Chief Scientific Advisor & inventor of the iLite® technology at Svar Life Science, highlights how cell-based assays are helping the development of complex biotherapeutics. The article also presents how this advanced engineering platform works…Read more & download
Behind the scenes at Novartis – The development of robust cell-based assays for gene therapy
In a recent editorial article, Dr Harald Messer, Gene Therapies Senior Scientist at Novartis, talks about the key considerations and challenges associated with developing biologically relevant cell-based assays that reflect mechanisms of action (MoA) and drug potency that represent the functionality and safety of gene therapy products.
The article also deals with the need for robust, customized assays in gene therapy, key considerations for potency testing, and much more…Read more & download
How biosimilars are expanding options for patient care
 In this editorial article, Heather Watson, Senior Manager in the Immunochemistry Department at Labcorp, highlights how the industry improves patient care by delivering innovative solutions that support faster and more cost-effective treatment.
In this editorial article, Heather Watson, Senior Manager in the Immunochemistry Department at Labcorp, highlights how the industry improves patient care by delivering innovative solutions that support faster and more cost-effective treatment.
The article also deals with the regulatory challenges in biosimilar development and how to address those challenges through in-house efforts or by partnering with other organizations offering specialized services and products in this area, such as Labcorp and Svar Life Science…Read more & download
 In this editorial article, Dr Niklas Finnberg & Dr Dorota Klaczkowska – Experts from Xbrane – discuss the biosimilar development process and how bioassays are the driving force behind reduced cost and accelerated timelines.
In this editorial article, Dr Niklas Finnberg & Dr Dorota Klaczkowska – Experts from Xbrane – discuss the biosimilar development process and how bioassays are the driving force behind reduced cost and accelerated timelines.
They also discuss the biosimilar development process, the integral role of bioassays in their work and the potential of biosimilars to drive a future of more equitable healthcare systems…Read more & download
 In this editorial article, Prof Reinhard Würzner – an internationally renowned expert – gives his valuable perspective on how the complement system can be used to detect and treat infectious diseases.
In this editorial article, Prof Reinhard Würzner – an internationally renowned expert – gives his valuable perspective on how the complement system can be used to detect and treat infectious diseases.
He also talks about how immunoassays have enabled advances in research, diagnosis, and therapy management concerning complement pathways. Furthermore, it presents the pan-European initiative project called Complement Regulation and Variations in Opportunistic Infections (CORVOS)…Read more & download
Novel COVID-19 biomarkers – The role of complement C4d & calprotectin
 In this editorial article, Dr Marie-Agnès Dragon-Durey highlights the role of complement C4d and calprotectin in detecting, diagnosing and treating viral infectious diseases.
In this editorial article, Dr Marie-Agnès Dragon-Durey highlights the role of complement C4d and calprotectin in detecting, diagnosing and treating viral infectious diseases.
The complement system is an important part of the innate immune response and plays a key role in the defense against invading pathogens, including viruses, bacteria, fungi and parasites. Recent studies have shown that complement overactivation plays a critical role in the pathogenesis of COVID-19 and other viral diseases, suggesting its huge potential as a biomarker for infectious diseases…Read more & download
Researchers develop a promising new therapy to tackle metabolic liver disease, NASH

In this editorial article, Dr Erik Tillman, Principal Scientist at Akero Therapeutics, describes his company’s quest to find an effective treatment for non-alcoholic steatohepatitis and how the functional iLite assay-ready cells they use deliver robust, reproducible and convenient ready-to-use cells that accelerate their research and support project goals.
The article also deals with the challenges that a virtual company faces and how forging relationships with trusted contract research organizations, like Svar Life Science, can ease and speed up the development process…Read more & download
Factors affecting the immunogenicity of biopharmaceuticals
 Immunogenic responses, where antibodies are produced by the immune system as a protective response, can adversely affect the pharmacokinetics, bioavailability, and efficacy of biopharmaceuticals, and may even neutralize the activity of the drug. Many different factors influence immunogenicity…Read more & download
Immunogenic responses, where antibodies are produced by the immune system as a protective response, can adversely affect the pharmacokinetics, bioavailability, and efficacy of biopharmaceuticals, and may even neutralize the activity of the drug. Many different factors influence immunogenicity…Read more & download
 The complement system is a complex hub-like network of proteins, reactions and processes. More than 50 fluid-phased, cell-surface associated and intra-cellular proteins function together to maintain immunosurveillance and homeostasis of our body.
The complement system is a complex hub-like network of proteins, reactions and processes. More than 50 fluid-phased, cell-surface associated and intra-cellular proteins function together to maintain immunosurveillance and homeostasis of our body.
Register to receive your free poster showing the complex hub-like network of the complement system pathways…Read more & download
 In this application story, we take a closer look at the results of the Svar Complement Assays when analyzing how the complement system is affected by a drug candidate.
In this application story, we take a closer look at the results of the Svar Complement Assays when analyzing how the complement system is affected by a drug candidate.
We showcase results from the study of the inhibitory effect of eculizumab on the complement system using two biomarker assays, one cell-based assay and the functional Wieslab assays, and how these assays are powerful tools for analyzing the effects of complement targeting drug candidates…Read more & download
How to design potency assays for gene therapy projects
 Learn more about the process at Svar of designing, constructing, producing and validating a fit-for-purpose potency assay for use in the batch release of an AAV-based gene therapy drug. In this case story, we describe the process of developing an advanced potency assay for an advanced product, like the reporter-gene assay – from the initial contact with the client through development and validation to the manufacturing of the finished product…Read more & download
Learn more about the process at Svar of designing, constructing, producing and validating a fit-for-purpose potency assay for use in the batch release of an AAV-based gene therapy drug. In this case story, we describe the process of developing an advanced potency assay for an advanced product, like the reporter-gene assay – from the initial contact with the client through development and validation to the manufacturing of the finished product…Read more & download
A cell-based ADCC bioassay comparison
 The surge of interest in developing therapeutic antibodies in the last few years has led to increased demand for reliable cell-based assays to assess the ability of these antibodies to elicit ADCC.
The surge of interest in developing therapeutic antibodies in the last few years has led to increased demand for reliable cell-based assays to assess the ability of these antibodies to elicit ADCC.
In this White Paper, we present data on the quantification of ADCC using cell-based iLite ADCC bioassays. In addition, we compare this technology to a traditional method based on NFAT effector cells and wild-type target cells…Read more & download
Bispecific antibodies – How to quantify their activity
 Bispecific antibodies (BsAbs) are used to treat many conditions and there is great hope that new antibody therapies will help in the treatment of many other diseases, including cancers, autoimmune disorders and infectious diseases in the future.
Bispecific antibodies (BsAbs) are used to treat many conditions and there is great hope that new antibody therapies will help in the treatment of many other diseases, including cancers, autoimmune disorders and infectious diseases in the future.
Download the White Paper using the form and learn how BsAbs work, how they are useful and how cell-based reporter-gene assays can be used as valuable tools in developing new therapeutic BsAbs…Read more & download
Designing optimal bioassays for measuring ADCC & ADCP activity
 Over the last decades, the interest in therapeutic monoclonal antibody (mAb) development has led to a need for sensitive, reliable, and biologically relevant bioassays for measuring Fc-effector activity.
Over the last decades, the interest in therapeutic monoclonal antibody (mAb) development has led to a need for sensitive, reliable, and biologically relevant bioassays for measuring Fc-effector activity.
Download the White Paper to learn about Fc-effector activity bioassay design and how the iLite® assays have been designed to offer a powerful and convenient way of measuring ADCC and ADCP activity while minimizing the disadvantages usually associated with reporter-gene assays…Read more & download
ON-DEMAND WEBINAR: Advancements in GMP QC Testing: A Comprehensive Guide to Cell-Based Assays
 Gain access to the on-demand version of the webinar and hear from experts on the latest cell-based technology and some important aspects of GMP requirements for bioassays. You’ll learn how these cell-based assays can support the drug development continuum, including GMP QC testing. Register to watch on-demand
Gain access to the on-demand version of the webinar and hear from experts on the latest cell-based technology and some important aspects of GMP requirements for bioassays. You’ll learn how these cell-based assays can support the drug development continuum, including GMP QC testing. Register to watch on-demand
Functional assays for biopharmaceutical development success
 Tune in to this webinar to learn how reporter-gene assays, combined with complement system assays, can be used to determine drug mechanisms of action and the expected immune response. In this webinar, we explain how functional assays can be used in safe and compliant biopharmaceutical development…Register to view the webinar
Tune in to this webinar to learn how reporter-gene assays, combined with complement system assays, can be used to determine drug mechanisms of action and the expected immune response. In this webinar, we explain how functional assays can be used in safe and compliant biopharmaceutical development…Register to view the webinar
Design of biosimilar bioanalytical programs: PK assay considerations
 Biosimilars are developed under the ‘totality of evidence’ principle, which can be defined as the sum of data from analytical, preclinical, and clinical studies. There is no “one size fits all’ approach to biosimilar product development.
Biosimilars are developed under the ‘totality of evidence’ principle, which can be defined as the sum of data from analytical, preclinical, and clinical studies. There is no “one size fits all’ approach to biosimilar product development.
There are numerous challenges with BIOSIM development. This online presentation will discuss some of those considerations, specifically focusing on the bioanalytical strategies applicable for PK assays…Register to view the webinar
 This scientific talk presents how the iLite® Reporter Cell Platform for assessing anti-AAV NAbs is a novel tool for rapid and reliable detection of an inhibitory humoral response to the viral vector and was developed to be used in clinical applications.
This scientific talk presents how the iLite® Reporter Cell Platform for assessing anti-AAV NAbs is a novel tool for rapid and reliable detection of an inhibitory humoral response to the viral vector and was developed to be used in clinical applications.
In this webinar, we present a novel two-component iLite® reporter cell system for detecting and quantifying neutralizing antibodies against AAV vectors…Register to view the webinar
Quantification of the neutralizing antibody response to recombinant AAV vectors
 This scientific talk, presented at PEGS 2021, presents a highly sensitive iLite® reporter assay, providing a highly sensitive, rapid, precise method for quantifying NAb response to a wide range of recombinant AAV vectors.
This scientific talk, presented at PEGS 2021, presents a highly sensitive iLite® reporter assay, providing a highly sensitive, rapid, precise method for quantifying NAb response to a wide range of recombinant AAV vectors.
A highly sensitive iLite® reporter assay based on a packaging cell line expressing a recombinant virus containing a tag sequence within the ITRs and a serotype-specific cap gene recognized specifically by a reporter cell line stably transfected with a luciferase reporter-gene placed under the control of an AAV-responsive chimeric promoter will be described…Register to view the webinar
The immune response to AAV gene therapy
 Dr Michael Tovey guides us through the challenges of accurately quantifying the immune response to AAV-mediated gene therapy. Adeno-Associated Virus (AAV) is the most common vector used to deliver the genetic cargo in gene therapy. Although powerful and efficient, this technique requires that the patient has not previously been subjected to the virus and has developed antibodies against the vector.
Dr Michael Tovey guides us through the challenges of accurately quantifying the immune response to AAV-mediated gene therapy. Adeno-Associated Virus (AAV) is the most common vector used to deliver the genetic cargo in gene therapy. Although powerful and efficient, this technique requires that the patient has not previously been subjected to the virus and has developed antibodies against the vector.
In this presentation, Dr Tovey presents a rapid and sensitive iLite reporter gene assay that is used for quantifying the immune response to a wide range of recombinant AAV vectors…Register to view the webinar
Overcoming Challenges in the Development of Potency Assays
 Quantification of Products Encoded by Recombinant AAV Vectors Expressing Transgenes Regulated by Retina-Specific Promoters
Quantification of Products Encoded by Recombinant AAV Vectors Expressing Transgenes Regulated by Retina-Specific Promoters
There is great interest in the development of gene therapy for inherited and degenerate disorders of the retina. However, in order to avoid adverse effects, it is important that the expression of the transgene is limited to its intended tissue. One way of achieving this is to regulate the transcription of the transgene using a cell-specific promoter, e.g., a promoter exclusively found in the cells of the cones or rods. Designing potency assays for gene therapy based on retina-specific promoters can be challenging…Register to view webinar
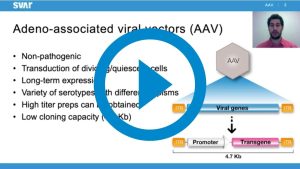 This scientific talk, Jordi Rodó, Ph.D., presents how the iLite® Reporter Cell Platform can be used for assay development within the field of AAV therapies and how iLite cell lines can be engineered for specific and general assays. He also present different strategies for the development of reliable bioassays for specific or more global use by showcasing a selection of our engineered iLite® cells.
This scientific talk, Jordi Rodó, Ph.D., presents how the iLite® Reporter Cell Platform can be used for assay development within the field of AAV therapies and how iLite cell lines can be engineered for specific and general assays. He also present different strategies for the development of reliable bioassays for specific or more global use by showcasing a selection of our engineered iLite® cells.
Svar Life Science today announced the adoption of a new advanced technology to enable the use of the Olink® high-throughput proteomics platform together with Svar CRO and its Biomarker Discovery Services.
The integration of Olink’s Novel Proteomics Solutions Technology will initially leverage high-throughput multiplex biomarker analysis to provide Svars’ clients with specific, precise…Read Press Release
 Svar Life Science Announces Launch of GMP QC Testing Services
Svar Life Science Announces Launch of GMP QC Testing Services
At Svar, we are committed to providing our customers with the highest quality products and services. Therefore, we are delighted to announce an expansion of our biologics testing capacity – the launch of our GMP QC testing services!
This new testing service offers a comprehensive approach to Bioassays and GMP potency testing to ensure the highest standards of QC services. With our GMP-certified lab and highly trained technicians, you can trust that your biopharmaceuticals are tested accurately and reliably…Read Press Release
Please visit our event page for more information about Svar Life Science at each conference.
This is the list of conferences Svar Life Science plans to attend for 2024. We hope to see you soon!
MARCH
March 6-8 | Copenhagen
March 11-13 | Los Angeles
APRIL
April 4-5 | Madrid
April 10-12 | Munich
DGfI- The German Society for Immunology – Complement System Meeting
April 15-17 | Gaithersburg-Washington
April 22-24 | Lisbon
Open Scientific EIP Symposium on Immunogenicity of Biopharmaceuticals
April 25-26 | London
MAY
May 7-11 | Baltimore
ASGCT – American Society of Gene & Cell Therapy
JUNE
June 4-6 | London
Antibody Engineering & Therapeutics Europe
June 11-16 | Loutraki, Greece
International Conference on Complement Therapeutics
June 20-21 | Montpellier, France
Antibody Industrial Symposium (AIS)
JULY
July 16-18 | Boston, USA
SEPTEMBER
September 2-6 | Lübeck, Germany
EMCHD – European Meeting on Complement in Human Diseases
September 17-19 | Malmö
NLS – Nordic Life Science Days
September 25-27 | Prague – Czech Republic
OCTOBER
October 2-4 |Boston
World Bispecific Antibody Summit
October 15-18 | Washington, USA
Immunogenicity & Bioassay Summit
October 10-12 | Phoenix, Arizona
Cell & Gene Meeting on the Mesa
October 20-23 | Salt Lake City, Utah
AAPS – American Association of Pharmaceutical Scientists
Paris, France
Pharmaceutical Science and Drug Manufacturing and Development
October 22-25 | Rome
ESGCT – European Society of Gene & Cell Therapy
NOVEMBER
November 20-22 | Barcelona, Spain
EBF – European Bioanalysis Forum
DECEMBER
December 1-31 | USA
Antibody Engineering & Therapeutics US
December, 1-31 | Boston, USA
HW 8th Complement-Based Drug Development Summit