Flow cytometric immunophenotyping of PBMCs and quantification of cytokine production from patients in a peptide vaccine study
Abstract
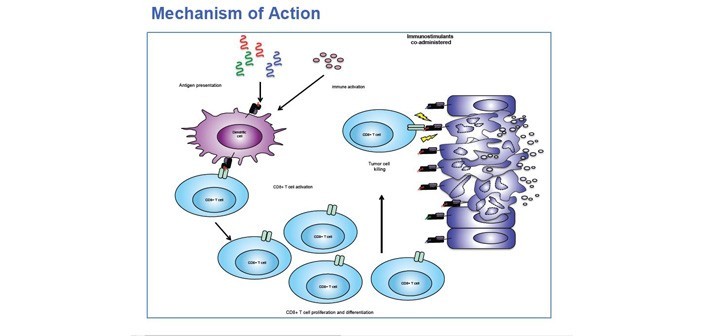
KCAS, LLC optimized a primary and secondary stimulation assay of Peripheral Blood Mononuclear Cell (PBMC)
samples from a clinical trial in order to characterize the immune response to a multi-valent peptide vaccine. In partnership with our pharmaceutical sponsor, an agreed upon set of samples were tested by KCAS, LLC to optimize a culture method, culture time course, stimulation, and re-stimulation. These samples were also used to optimize surface and intracellular staining of the following panel: CD45, CD3 CD8, PD1, CCR7, IFNγ, TNFα, IL-2 and viability dye. Upon review of the initial optimized method, the full cohort of patient samples were cultured, treated, stained, and analyzed.
Subsequent data analysis of PBMC response to stimulation/re-stimulation, as measured by production of TNFα and IFNγ, provide good translational support to clinical data. Additional testing continues in order to further assess individual peptide responses.